
If you are redistributing all or part of this book in a digital format, Then you must include on every physical page the following attribution: If you are redistributing all or part of this book in a print format, Want to cite, share, or modify this book? This book uses the (d) The paths followed by the α particles match the predictions from (a), (b), and (c). If the nucleus is larger, the positive charge is larger and the expected deflections are larger-more α particles will be deflected, and the deflection angles will be larger. (c) If the nucleus is smaller, the positive charge is smaller and the expected deflections are smaller-both in terms of how closely the α particles pass by the nucleus undeflected and the angle of deflection. (b) Higher-energy α particles that pass near the nucleus will still undergo deflection, but the faster they travel, the less the expected angle of deflection. The more directly toward the nucleus the α particles are headed, the larger the deflection angle will be. Those α particles that pass near the nucleus will be deflected from their paths due to positive-positive repulsion. Below is an example of the element carbon on the periodic table.(a) The Rutherford atom has a small, positively charged nucleus, so most α particles will pass through empty space far from the nucleus and be undeflected. All of the elements that are in the same period contain differing numbers of electrons in the same outer shell. Each member of the group has its outermost electrons in different shells. They all contain the same number of electrons in their outermost shell. All of the elements that are part of the same group have similar chemical properties. Elements are arranged in columns, or groups, and rows, or periods, in the periodic table. All elements are organized on a periodic table of elements. Therefore, as a general rule, an atom's atomic number is also equal to the number of electrons within that atom. Atoms that are "free" or uncharged have the same number of electrons as protons. All atoms with six protons are classified as carbon atoms, whereas all oxygen atoms have eight protons. An element's atomic number distinguishes it from other elements. Each atomic number corresponds to a single element.

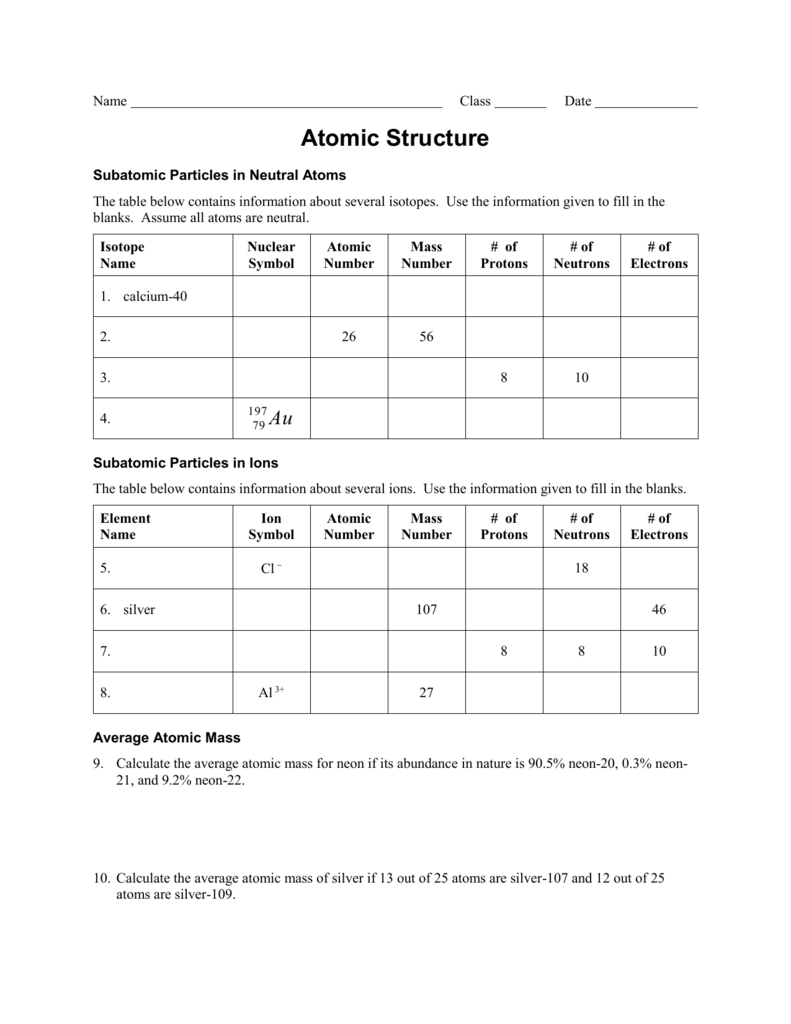
The number of protons determines the atom's atomic number. The nucleus of an atom has a specific number of protons. It is this attraction that keeps the electrons inside the atom.Ģ Scientists use characteristics of atoms to describe elements. Since electrons are negatively charged and opposite charges attract, the electrons are attracted to the nucleus, which has a positive charge. Each shell can only have a certain number of electrons.
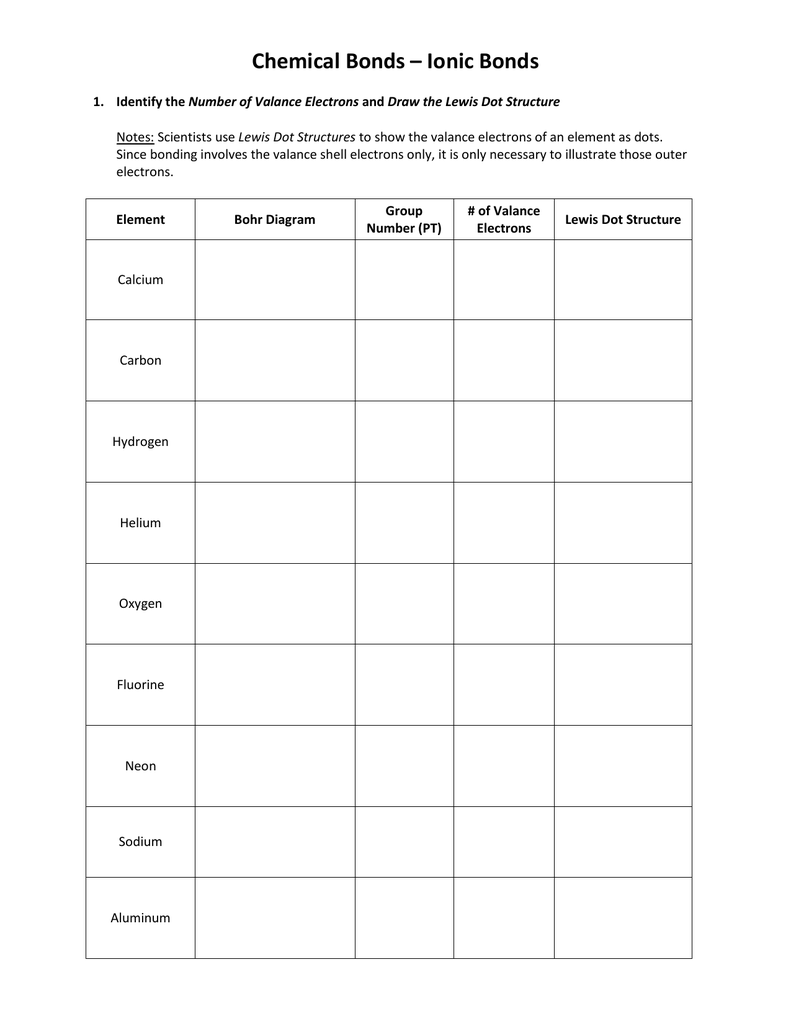
These areas of electrons are called energy levels or shells.
Within this electron cloud, the electrons are spaced at different distances from the nucleus. Fast-moving electrons form a cloud around the nucleus. Most of the atom's volume is empty space.

However, the nucleus's size is extremely small compared to the size of the atom. Most of the atom's mass is found in the nucleus. The nucleus is made up of protons and neutrons (picture red and green gumballs stuck together) with electrons moving at high speeds around the outside of the nucleus (imagine gumballs on a circular wire). Print Earth Lab: Atomic Structure (font options, pick words for additional puzzles, and more)ġ When scientists design models of atoms, they usually show a simplified version of the atom's nucleus and its subatomic particles. Uncharged, subatomic, atomic, periodic, proton, neutron, collection, element, account, define, column, volume, atom, fast-moving, gumballs, naturally Worksheets and No Prep Teaching Resources


 0 kommentar(er)
0 kommentar(er)
